Cleanrooms play a critical role in the labs and professions that rely on them. Industries such as life sciences, electronics, pharmaceuticals, and food manufacturing require highly controlled environments to refine products and conduct research without contamination. Airborne particles, dust, and other environmental contaminants can compromise the integrity of research, experiments, or production processes. Cleanrooms solve this problem by providing a space that minimizes contaminants and strictly regulates conditions to meet precise industry standards.
Often called “controlled environments,” cleanrooms actively restrict the introduction of pollutants. They also maintain consistent airflow, temperature, and humidity. Features such as laminar airflow systems, entryway air showers, sticky mats, and strict employee protocols help maintain cleanliness. The design and construction of a cleanroom—its walls, ceilings, and floor surfaces—further reduce contamination. However, no matter how sophisticated the design, technicians must perform ongoing cleanroom testing and certification in New York to ensure compliance with industry standards and optimal performance.
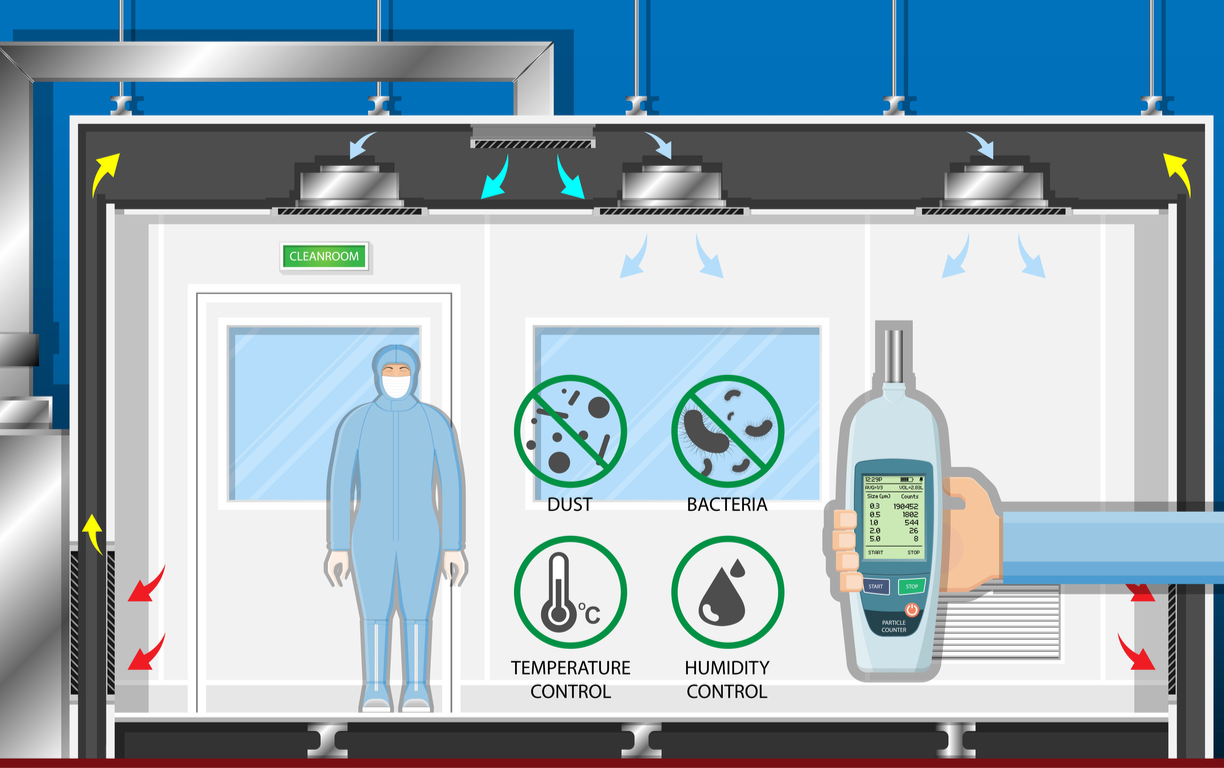
Understanding Cleanroom Testing
Cleanroom testing plays a critical role in evaluating whether all environmental control systems and features function correctly. ISO-14644-1 standards classify cleanrooms from ISO Class 1 to ISO Class 9 based on the allowable number of particles per cubic meter. This classification guides the testing process, helping technicians measure and evaluate the performance of each cleanroom feature accurately.
Every cleanroom relies on a combination of components to maintain a contamination-free environment. HEPA filtration, laminar airflow, and entryway air showers serve as standard features, while many advanced cleanrooms include additional specialized equipment. Technicians systematically measure airflow, filtration efficiency, and particle counts to verify that the environment meets both industry and operational requirements. Proper testing protects both products and personnel by ensuring cleanroom operations remain uncompromised.
Airflow Volume and Velocity Testing
Airflow testing is fundamental to evaluating a cleanroom’s effectiveness. Technicians measure airflow patterns—both unidirectional and non-unidirectional—to confirm that air moves consistently throughout the room. Using instruments such as anemometers or airflow meters, technicians measure air velocity and calculate the air exchange rate. Accurate measurements verify that the airflow is sufficient to push particles out of the controlled space, preventing contamination.
In addition to velocity, airflow volume tests are performed using a volume flow hood. This device captures air exiting from each filter or diffuser, allowing technicians to assess whether the air distribution covers the entire cleanroom. A properly functioning airflow system ensures that air is continuously filtered, circulated, and expelled, creating an environment that meets stringent ISO standards.
HEPA Filter Integrity Testing
HEPA filters are vital for cleanrooms, capable of removing nearly all airborne particles above 5µm. For smaller particles in the range of 0.15µm to 0.25µm, HEPA filters typically remove between 95% and 99.9995%. The grade and efficiency of the filter directly affect the cleanroom’s ability to maintain a particle-free environment.
During HEPA filter integrity testing, technicians check for leaks, tears, or other damages. Even minor imperfections can compromise the entire cleanroom. Specialized equipment such as photometers or aerosol generators is used to detect leaks and confirm the filter’s effectiveness. A cleanroom passes this critical test only when all filters are performing as required, ensuring that airborne contamination is effectively minimized.

Additional Cleanroom Tests
Beyond airflow and filtration testing, additional evaluations are critical for comprehensive cleanroom testing and certification in New York. Non-viable particle counting involves sampling airborne particles to confirm they remain within acceptable limits for the room’s ISO classification. Pressurization testing ensures proper positive or negative pressure is maintained between cleanroom zones, preventing the influx of contaminants.
Other assessments may include temperature and humidity monitoring, airflow visualization tests using smoke or fog, and surface particle counts. Collectively, these tests provide a detailed understanding of how well a cleanroom performs and whether it maintains the necessary environment for sensitive processes. Technicians carefully document results and compare them to ISO and cGMP standards to determine compliance.
Cleanroom Certification in New York
Cleanroom certification is the formal confirmation that a cleanroom meets the required operational and safety standards. Initial certification typically occurs after construction or renovation, verifying that the cleanroom meets ISO specifications and client-specific requirements. Certification is not a one-time process; recurring evaluations, often conducted annually or semi-annually, ensure continued compliance and consistent performance.
During certification, technicians assess all previous testing data, review any modifications or updates to the cleanroom, and perform the same rigorous testing protocols. For instance, renovations, equipment upgrades, or contamination events may necessitate re-certification. Each certification report includes detailed findings, outlining airflow measurements, particle counts, filter performance, and other critical metrics. Maintaining certification is essential for regulatory compliance, quality control, and uninterrupted operations in labs and manufacturing facilities.
Technicians performing certification follow current good manufacturing practices (cGMP) and adhere to guidelines from the Institute of Environmental Sciences and Technology (IEST) and the International Organization for Standardization (ISO). By following these standards, certification provides a trusted benchmark of cleanroom performance that can be relied upon by auditors, inspectors, and internal quality teams.
Documentation and Compliance
An integral component of cleanroom certification is proper documentation. Comprehensive records demonstrate compliance with regulatory bodies, provide traceability for audits, and support internal quality assurance programs. Certification reports typically include:
- ISO classification and particle count results
- Airflow velocity and volume data
- HEPA filter inspection and test results
- Pressurization and environmental condition data
- Recommendations for corrective actions, if any
Maintaining detailed records ensures ongoing regulatory compliance and facilitates future certifications. Technicians schedule recurring evaluations to minimize disruption to operations, ensuring that cleanrooms continue to operate at optimal efficiency with minimal downtime. In New York, where labs and manufacturers face strict regulatory oversight, maintaining accurate records is as crucial as the testing and certification itself.
Certification Professional Management Program
To streamline ongoing compliance, many facilities implement a structured program for scheduled testing and certification. A Certification Professional Management Program organizes recurring assessments, ensuring that each unit and component is evaluated at the correct intervals. Such programs are designed to prevent lapses in certification, reduce operational interruptions, and maintain continuous compliance with ISO and cGMP standards.
Facilities benefit from a structured program by receiving timely notifications of upcoming tests, scheduled maintenance, and any necessary adjustments to equipment. By proactively managing certifications and maintenance, cleanrooms consistently meet industry standards while minimizing the risk of contamination or downtime. This systematic approach is particularly valuable in New York, where regulatory inspections can be rigorous and frequent.
Importance of Minimizing Downtime
Downtime in cleanrooms can disrupt research, manufacturing, and product development. Effective cleanroom testing and certification practices aim to minimize downtime while maintaining compliance. Technicians often use readily available replacement parts and flexible scheduling to ensure equipment is operational as quickly as possible. By combining rapid response with careful, systematic testing, facilities maintain high productivity without compromising safety or cleanliness standards.
Conclusion
Cleanrooms are vital for industries that require controlled, contamination-free environments. Regular cleanroom testing and certification in New York ensures that these facilities maintain compliance with ISO-14644 standards, cGMP regulations, and industry-specific requirements. Comprehensive testing—including airflow, HEPA filtration, particle counting, and pressurization—verifies the performance of each cleanroom feature, while certification provides official documentation of compliance and operational integrity.
Structured programs for recurring testing, maintenance, and certification help facilities stay ahead of regulatory requirements, minimize downtime, and maintain optimal performance. Whether during initial certification, post-renovation evaluation, or scheduled annual inspections, a robust cleanroom testing and certification protocol is essential for ensuring safety, reliability, and efficiency in laboratories and production facilities across New York.
Ultimately, cleanroom testing and certification are not just procedural requirements—they are integral to maintaining the quality, safety, and success of the industries that rely on controlled environments every day.